1. What is the projected Compound Annual Growth Rate (CAGR) of the Cell Therapy Regenerative Medicine?
The projected CAGR is approximately 21.4%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Cell Therapy Regenerative Medicine
Cell Therapy Regenerative MedicineCell Therapy Regenerative Medicine by Type (NK Cell Therapy, CAR-T Cell Therapy, Others, World Cell Therapy Regenerative Medicine Production ), by Application (Hospital, Medical Research Center, Others, World Cell Therapy Regenerative Medicine Production ), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2026-2034
The cell therapy regenerative medicine market is poised for substantial expansion, propelled by the escalating incidence of chronic diseases, groundbreaking advancements in cell therapy technologies, and increased investments in research and development. This dynamic sector is projected to witness a compound annual growth rate (CAGR) of 21.4%, with the market size anticipated to grow from $24.39 billion in the base year 2025 to a significantly larger figure by 2033. The success of clinical trials for diverse conditions, including cardiovascular and neurological disorders, alongside the development of innovative cell-based therapies like CAR T-cell therapy, are key growth drivers. Leading companies such as DePuy Synthes, Medtronic, and Zimmer Biomet are actively influencing this market through strategic collaborations, acquisitions, and the introduction of novel products.
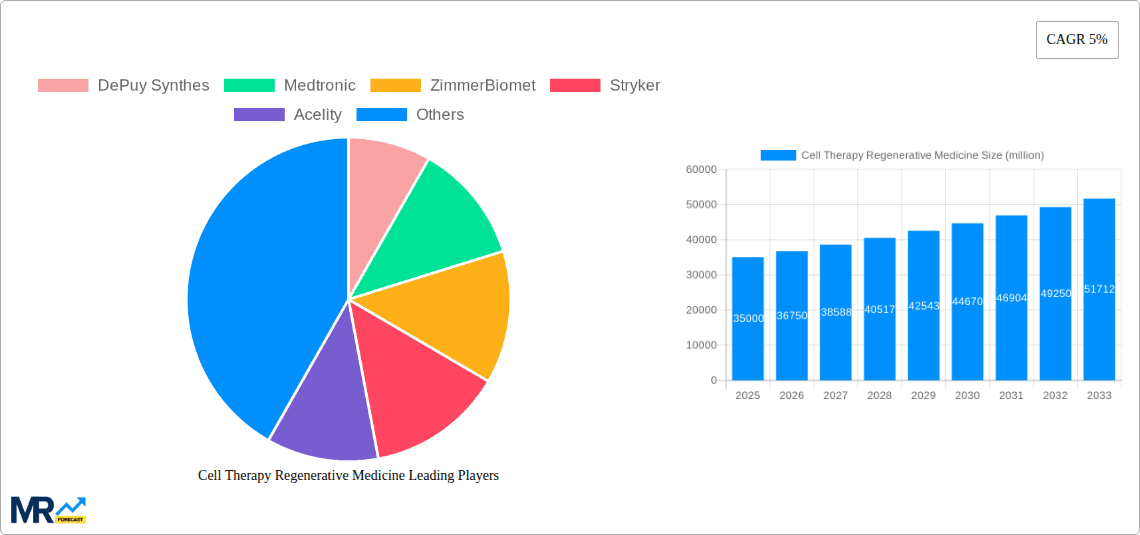

Key challenges, including stringent regulatory pathways and elevated manufacturing costs, persist. The intricate nature of cell therapy production necessitates rigorous quality control, contributing to higher expenses. Navigating the regulatory approval process for new therapies can also be time-consuming. However, ongoing efforts to develop cost-efficient manufacturing solutions and streamline regulatory frameworks are expected to mitigate these hurdles. Future market segmentation will likely highlight significant variations across distinct cell types and therapeutic applications. Geographically, North America and Europe are anticipated to lead market dominance, with gradual expansion into Asia-Pacific and other emerging economies. The persistent focus on personalized medicine and the increasing adoption of advanced cell therapy techniques underscore the continued growth trajectory of this critical field within regenerative medicine.
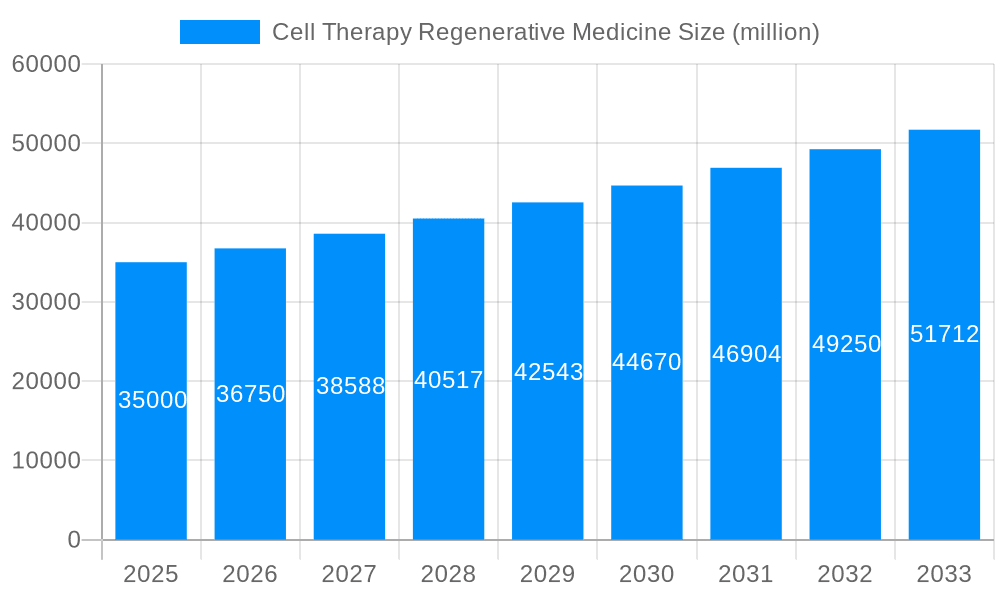

The cell therapy regenerative medicine market is experiencing exponential growth, projected to reach multi-billion dollar valuations by 2033. Driven by an aging global population and increasing prevalence of chronic diseases requiring advanced therapeutic interventions, this sector showcases remarkable promise. The market's expansion is fueled by significant advancements in cell processing technologies, personalized medicine approaches, and a growing understanding of the body's regenerative capabilities. The estimated market value in 2025 is pegged at [Insert Estimated Value in Millions USD], a substantial increase from the historical period (2019-2024). This burgeoning field encompasses various therapeutic modalities, including stem cell therapy, gene therapy, and immunotherapy, each targeting a diverse range of conditions, from orthopedic injuries to autoimmune diseases and cardiovascular ailments. The forecast period (2025-2033) anticipates robust expansion, propelled by ongoing clinical trials demonstrating efficacy and safety across various applications. This expansive growth is further stimulated by the continuous influx of investments from both private and public sectors, emphasizing the high potential return on investment within this dynamic industry. The increasing collaborations between research institutions, pharmaceutical companies, and regulatory bodies are streamlining the regulatory pathways, accelerating the transition of innovative cell therapies from the laboratory to the clinic, thereby significantly impacting market growth projections. The key market insights reveal a shift towards personalized medicine strategies, as well as an increasing focus on developing cost-effective manufacturing processes to make these therapies more accessible. This dynamic landscape is characterized by intense competition among established players and emerging biotech companies, driving innovation and accelerating the development of advanced therapeutic options.
Several key factors are propelling the rapid expansion of the cell therapy regenerative medicine market. Firstly, the escalating global burden of chronic diseases, such as osteoarthritis, cardiovascular diseases, and neurodegenerative disorders, creates a significant unmet medical need. Cell-based therapies offer a potential cure or significant improvement for conditions previously considered incurable, driving substantial market demand. Secondly, technological advancements in cell processing and manufacturing are enhancing the efficiency, scalability, and safety of cell therapy production. This has led to a decrease in manufacturing costs, increasing accessibility and market penetration. Thirdly, the increasing adoption of personalized medicine approaches allows for tailoring cell therapies to individual patient characteristics, maximizing therapeutic efficacy and minimizing adverse effects. This precision medicine approach represents a paradigm shift in healthcare, bolstering the market's growth trajectory. Fourthly, supportive regulatory frameworks and increased investment in research and development (R&D) from both governmental agencies and private investors are fostering innovation and accelerating the commercialization of novel cell therapies. Finally, growing public awareness and patient advocacy are contributing to increased demand and acceptance of cell-based therapies as effective treatment options. These synergistic forces are collectively responsible for the observed exponential growth and future market potential of cell therapy regenerative medicine.
Despite its immense potential, the cell therapy regenerative medicine market faces several challenges. High manufacturing costs remain a significant hurdle, limiting accessibility and affordability for many patients. The complexity of cell processing and the need for specialized infrastructure contribute to these high costs. Moreover, the long and complex regulatory pathways for approval of novel cell therapies can significantly delay market entry and impact time-to-market. Rigorous clinical trials are essential to demonstrate both safety and efficacy, adding to the overall cost and time investment. Furthermore, ensuring the long-term viability and functionality of cells after transplantation remains a significant challenge, impacting the overall effectiveness of the therapy. The risk of immunological rejection or tumorigenicity is also a critical concern that requires ongoing research and technological advancements to mitigate effectively. Finally, inconsistencies in the manufacturing processes can lead to variability in the quality and potency of the final product. Addressing these challenges requires collaborative efforts between researchers, regulators, manufacturers, and healthcare providers to streamline the regulatory process, optimize manufacturing efficiencies, and develop more effective and safer cell therapy products, making them widely available to patients in need.
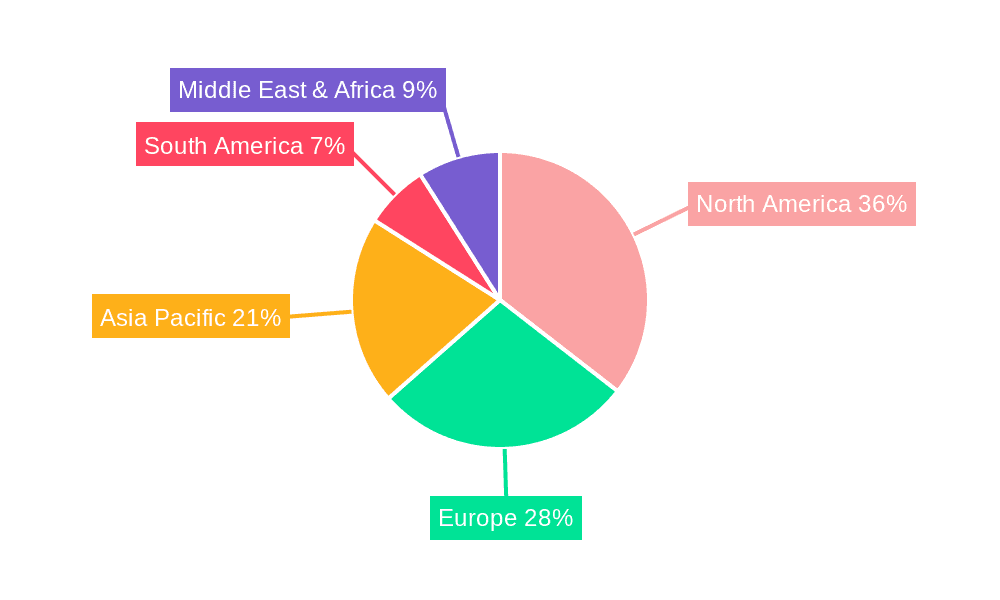
The global cell therapy regenerative medicine market is characterized by regional variations in growth and market share. North America, particularly the United States, currently holds a significant market share due to the presence of well-established biotechnology and pharmaceutical companies, robust regulatory frameworks, and high healthcare expenditure. Europe is another major player, exhibiting strong growth, fueled by significant investments in research and development and increasing adoption of advanced therapies. The Asia-Pacific region is projected to witness the fastest growth in the forecast period, driven by increasing healthcare awareness, rising disposable incomes, and a growing number of clinical trials. Within segments, stem cell therapy currently dominates the market due to its established clinical applications and extensive research. However, gene therapy and immunotherapy segments are rapidly gaining traction, exhibiting high growth potential.
The market dominance will likely shift over time, with the Asia-Pacific region expected to become a key player due to its substantial population and growing healthcare sector. Similarly, gene therapy and immunotherapy are poised to challenge the dominance of stem cell therapy as technological advancements continue to make these therapies increasingly effective and efficient.
The cell therapy regenerative medicine industry is experiencing significant growth fueled by a confluence of factors. The expanding prevalence of chronic diseases necessitates advanced therapeutic options, propelling demand. Simultaneously, breakthroughs in cell processing technologies are improving treatment efficacy and safety, widening market accessibility. Strategic collaborations between pharmaceutical companies and research institutions are accelerating the translation of research into commercial products, while supportive regulatory policies are creating a favorable environment for market expansion. These intertwined elements converge to cultivate a robust and dynamic market poised for continued expansion and innovation.
This report provides a comprehensive overview of the cell therapy regenerative medicine market, encompassing market size estimations, growth forecasts, key players analysis, and an in-depth assessment of the market’s driving forces, challenges, and future prospects. It includes detailed regional and segment analysis, highlighting areas of significant growth and potential. The report offers invaluable insights for stakeholders, including investors, researchers, pharmaceutical companies, and healthcare providers, involved in this rapidly evolving therapeutic area. This in-depth analysis equips readers with the knowledge necessary to navigate the complex landscape of cell therapy regenerative medicine and make informed decisions.

| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 21.4% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 21.4%.
Key companies in the market include DePuy Synthes, Medtronic, ZimmerBiomet, Stryker, Acelity, MiMedx Group, Organogenesis, UniQure, Cellular Dynamics International, Osiris Therapeutics, Vcanbio, Gamida Cell, Golden Meditech, Cytori, Celgene, Vericel Corporation, Guanhao Biotech, Mesoblast, Stemcell Technologies, Bellicum Pharmaceuticals, .
The market segments include Type, Application.
The market size is estimated to be USD 24.39 billion as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in billion and volume, measured in K.
Yes, the market keyword associated with the report is "Cell Therapy Regenerative Medicine," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Cell Therapy Regenerative Medicine, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.