1. What is the projected Compound Annual Growth Rate (CAGR) of the Cefalexin API?
The projected CAGR is approximately 5.6%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Cefalexin API
Cefalexin APICefalexin API by Type (>99.5, >99.8), by Application (Powder Injection, Injection), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2026-2034
The global Cefalexin API market, valued at $578.3 million in 2025, is projected to experience robust growth, driven by the increasing prevalence of bacterial infections requiring antibiotic treatment and a rising demand for affordable, effective therapies in both developed and developing nations. The market's Compound Annual Growth Rate (CAGR) of 5.6% from 2025 to 2033 indicates sustained expansion. Key drivers include the rising incidence of respiratory tract infections, skin and soft tissue infections, and urinary tract infections, all of which are commonly treated with Cefalexin. Furthermore, the increasing prevalence of antibiotic-resistant bacteria is indirectly boosting the market, as Cefalexin remains effective against certain strains where other antibiotics have failed. However, the market faces challenges from the growing emphasis on antibiotic stewardship and the development of newer, broader-spectrum antibiotics. Market segmentation reveals a higher demand for higher purity Cefalexin API (>99.8%), primarily driven by stringent pharmaceutical regulations and quality control standards. The powder injection application segment dominates, reflecting the widespread use of Cefalexin in injectable formulations. Major players like United Laboratories, Lupin Pharmaceuticals, and Hospira are actively shaping the market dynamics through their production capacity, research and development efforts, and global distribution networks. Regional variations exist, with North America and Europe holding significant market shares, driven by strong healthcare infrastructure and high pharmaceutical consumption. However, rapidly growing economies in Asia Pacific, particularly China and India, present significant growth opportunities due to increasing healthcare spending and rising infection rates. The continued focus on improving healthcare access and managing antibiotic resistance will play a crucial role in shaping the Cefalexin API market's trajectory over the forecast period.
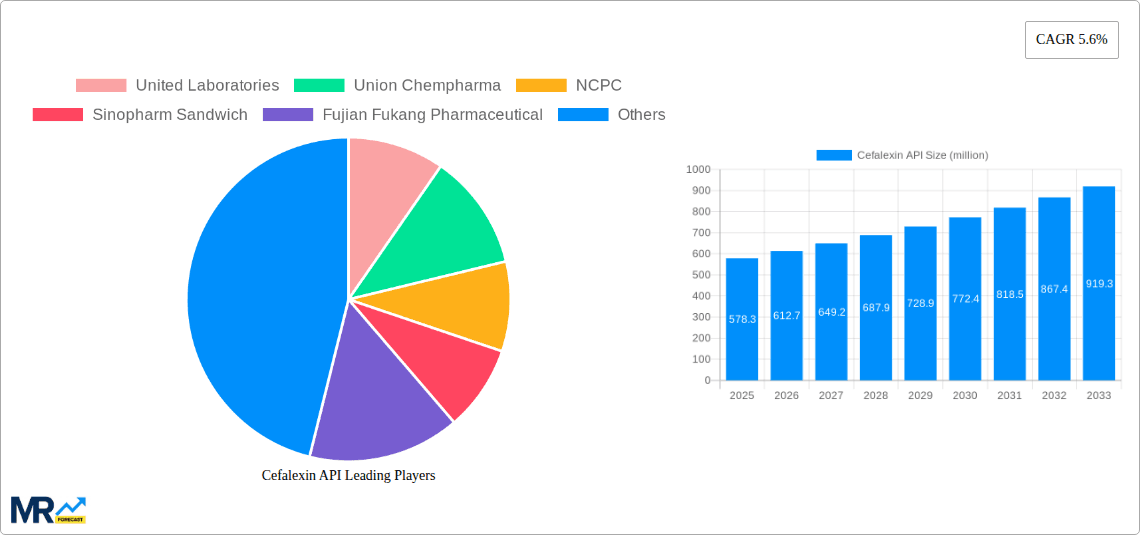

The competitive landscape is characterized by a mix of established multinational pharmaceutical companies and regional players. These companies employ various strategies, including mergers and acquisitions, strategic partnerships, and capacity expansion, to maintain their market positions. The success of these strategies depends on factors such as the ability to meet evolving regulatory requirements, invest in research and development, and maintain cost-competitiveness. The forecast period will likely witness increased focus on optimizing supply chains, improving production efficiency, and exploring new formulations and delivery systems to enhance the therapeutic effectiveness and patient compliance of Cefalexin. Further, the market is expected to see increased regulatory scrutiny on antibiotic manufacturing and distribution, emphasizing quality control and safety standards. Addressing these factors will be crucial for market players to ensure sustained profitability and secure long-term success within this dynamic market landscape.
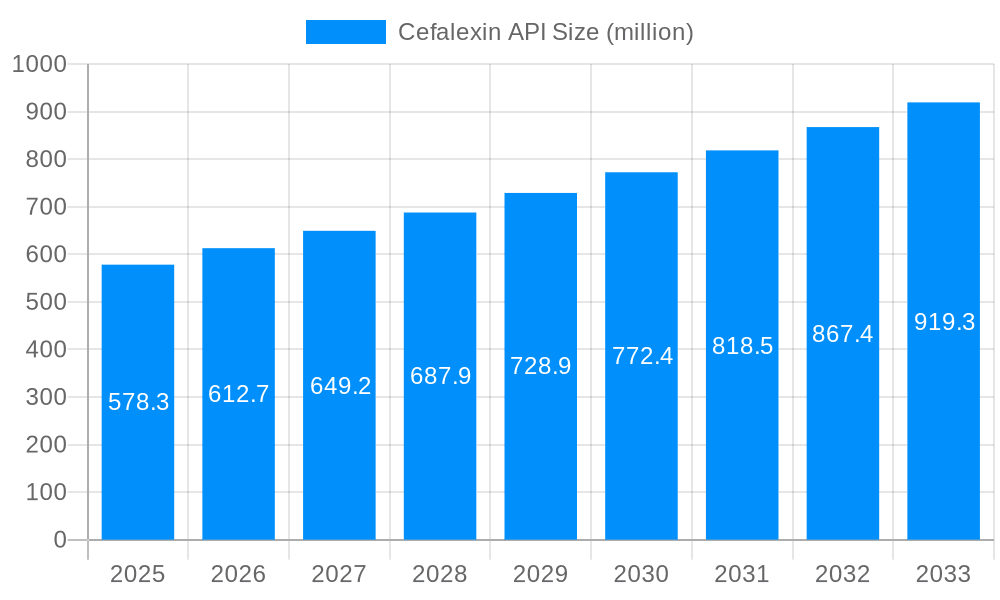

The global Cefalexin API market, valued at approximately $XXX million in 2025, is projected to witness robust growth throughout the forecast period (2025-2033). Driven by increasing demand for effective and affordable antibiotics, the market exhibits a positive trajectory. Analysis of historical data (2019-2024) reveals a consistent upward trend, with a notable acceleration in growth observed during the recent years. This growth is fueled by several factors, including rising prevalence of bacterial infections, expanding healthcare infrastructure in developing economies, and increasing government initiatives promoting accessible healthcare. The market is segmented by purity level (>99.5% and >99.8%) and application (powder injection and injection). While both purity levels are experiencing significant growth, the higher purity grade (>99.8%) is projected to capture a larger market share owing to its superior quality and suitability for a broader range of pharmaceutical applications. Similarly, the injectable formulations segment is expected to dominate the market due to the effectiveness and rapid action of intravenous administration, particularly in critical care settings. Key players like United Laboratories, Lupin Pharmaceuticals, and Sinopharm Sandwich are actively contributing to market growth through strategic expansions, collaborations, and continuous innovation in manufacturing processes. The market is characterized by both established players and emerging companies, leading to intense competition and driving innovation in terms of cost-effectiveness and product quality. This competitive landscape further fuels market expansion and provides consumers with diverse choices. The future of the Cefalexin API market appears promising, with growth anticipated across various geographic regions, reflecting the global need for affordable and effective antibiotic solutions. The report provides a detailed analysis of these trends, offering valuable insights for stakeholders in the pharmaceutical industry.
Several key factors contribute to the growth of the Cefalexin API market. Firstly, the rising prevalence of bacterial infections globally, including both community-acquired and hospital-acquired infections, necessitates increased production and distribution of Cefalexin, a widely prescribed first-line antibiotic. Secondly, the expanding healthcare infrastructure, particularly in emerging economies, enhances accessibility to quality healthcare, directly boosting the demand for essential antibiotics like Cefalexin. Governments are investing heavily in strengthening healthcare systems, which further fuels market growth. Thirdly, the relatively lower cost of Cefalexin compared to other broad-spectrum antibiotics makes it an attractive choice for healthcare providers and patients, particularly in resource-constrained settings. This affordability coupled with its effectiveness in treating various infections drives its widespread usage. Furthermore, ongoing research and development efforts focused on improving the manufacturing process and enhancing Cefalexin's efficacy further contribute to market expansion. Increased investment in research and development by key players is translating into the development of more effective formulations and improved production methods, thus reducing costs and improving efficiency. Finally, the growing awareness among healthcare professionals and the public about the importance of antibiotic stewardship and appropriate antibiotic use is inadvertently increasing the demand for reliable and quality-assured Cefalexin API.
Despite the promising outlook, the Cefalexin API market faces certain challenges. The primary concern is the increasing incidence of antibiotic resistance. The overuse and misuse of antibiotics, including Cefalexin, have led to the emergence of resistant bacterial strains, diminishing the effectiveness of the drug. This necessitates the development of new antibiotics and strategies to combat antibiotic resistance, creating a significant challenge for the industry. Fluctuations in raw material prices also affect the profitability of Cefalexin API manufacturers. The cost of raw materials used in the production of Cefalexin can significantly impact production costs and ultimately affect market prices. Regulatory hurdles and stringent quality control standards imposed by regulatory bodies can also pose challenges for manufacturers. Compliance with stringent Good Manufacturing Practices (GMP) and other regulations requires substantial investment and effort. Furthermore, intense competition among numerous Cefalexin API manufacturers leads to price pressure and a need for continuous innovation to maintain market share. This competitive landscape necessitates manufacturers to focus on cost optimization and improvement of product quality while maintaining profitability. Lastly, variations in demand across different geographic regions can pose challenges in terms of supply chain management and market forecasting.
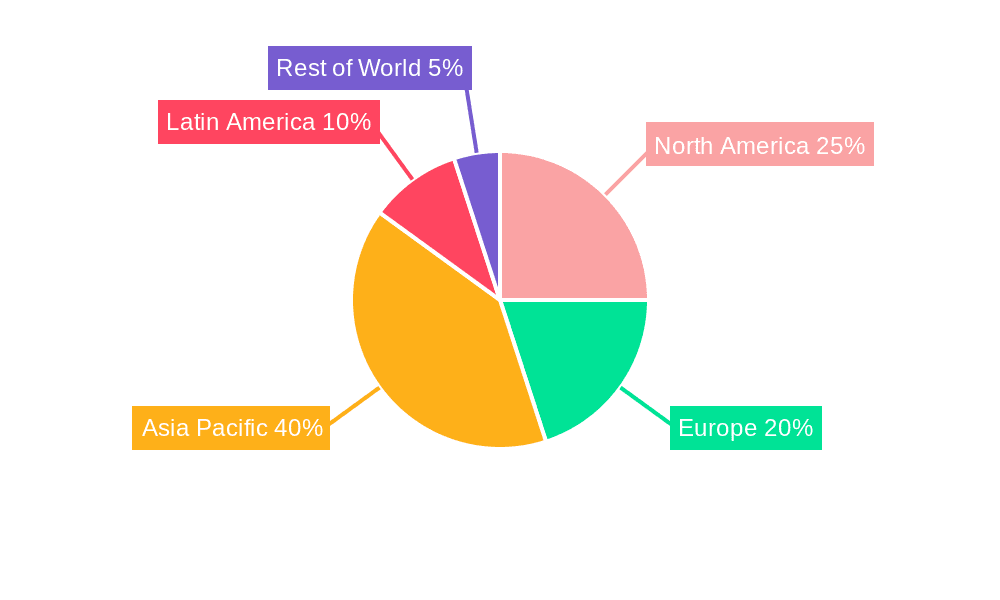
The Cefalexin API market demonstrates strong regional disparities. Asia-Pacific, particularly India and China, is expected to dominate the market due to the presence of a large number of pharmaceutical manufacturers, a substantial patient population, and increasing healthcare spending. Within the segments, the >99.8% purity Cefalexin API is projected to capture a larger market share than the >99.5% purity segment, primarily due to its superior quality and wider acceptance by pharmaceutical companies for various formulations. The injection application segment is also anticipated to dominate over the powder injection segment, driven by the higher demand for injectable formulations in hospitals and critical care settings.
The significant presence of numerous large-scale pharmaceutical manufacturers in Asia, particularly India and China, provides a significant advantage in terms of production capacity and cost-effectiveness. This, coupled with the region's high prevalence of bacterial infections and expanding healthcare infrastructure, translates into a consistently high demand for Cefalexin API. The higher purity grade (>99.8%) commands a premium due to its superior quality and suitability for diverse pharmaceutical preparations. Injectable formulations are preferred in clinical settings due to their rapid action and ease of administration, solidifying their dominance in the market. Therefore, the combination of regional factors and product attributes indicates a strong projection for the Asia-Pacific region and the higher purity, injection segment. This segment is poised for significant growth and is likely to remain the dominant force in the Cefalexin API market throughout the forecast period.
The Cefalexin API market is fueled by several growth catalysts. The rising prevalence of bacterial infections coupled with the increasing affordability and accessibility of healthcare services significantly contribute to the market's expansion. Technological advancements in manufacturing processes, leading to improved product quality and reduced production costs, also stimulate growth. Moreover, strategic collaborations and partnerships between manufacturers, research institutions, and regulatory bodies promote market development and support the introduction of new and improved formulations.
This report provides a comprehensive overview of the Cefalexin API market, encompassing historical data, current market dynamics, and future projections. It offers detailed insights into market trends, driving forces, challenges, key players, and significant developments. The report is an invaluable resource for businesses, investors, and researchers seeking a deep understanding of the Cefalexin API market landscape. The detailed segmental analysis and regional breakdown provide a granular perspective on the market dynamics, enabling informed strategic decision-making.

| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 5.6% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 5.6%.
Key companies in the market include United Laboratories, Union Chempharma, NCPC, Sinopharm Sandwich, Fujian Fukang Pharmaceutical, Youcare Pharmaceutical, Shanghai Pharmaceutical, Livzon Pharmaceutical, Qilu Antibiotics Pharmaceutical, Lupin Pharmaceuticals, Hospira, .
The market segments include Type, Application.
The market size is estimated to be USD 578.3 million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Cefalexin API," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Cefalexin API, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.