1. What is the projected Compound Annual Growth Rate (CAGR) of the Cardiovascular Disease Monitoring and Diagnostic Devices?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Cardiovascular Disease Monitoring and Diagnostic Devices
Cardiovascular Disease Monitoring and Diagnostic DevicesCardiovascular Disease Monitoring and Diagnostic Devices by Type (ECG Systems, Event Monitors, Holter Monitors, Implantable Loop Recorders, ECG Management Systems, World Cardiovascular Disease Monitoring and Diagnostic Devices Production ), by Application (Heart Disease, Coronary Heart Disease, World Cardiovascular Disease Monitoring and Diagnostic Devices Production ), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2026-2034
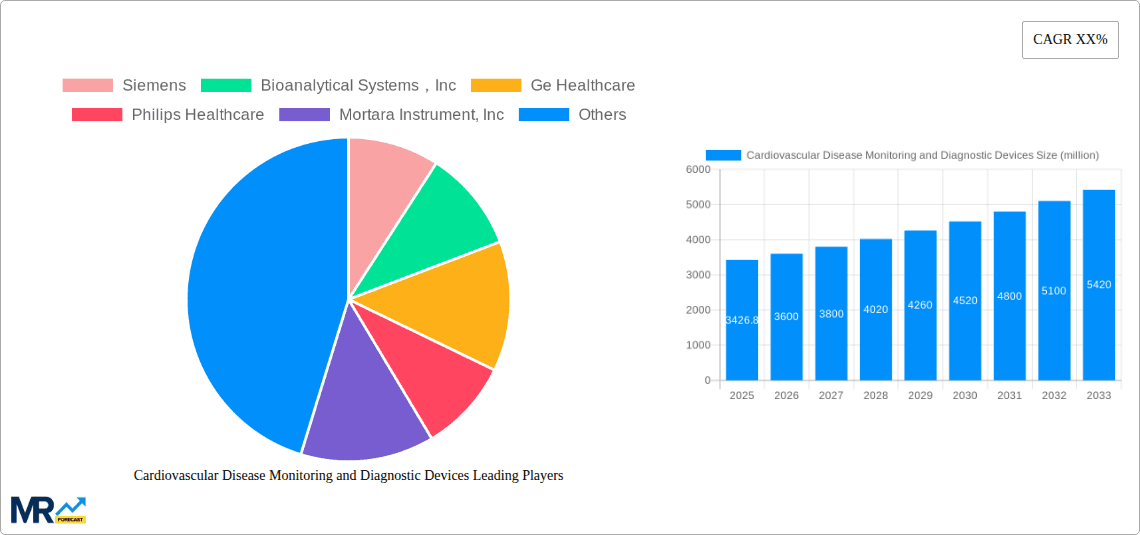
The global cardiovascular disease (CVD) monitoring and diagnostic devices market is a significant and rapidly expanding sector, projected to reach a substantial size driven by increasing prevalence of CVDs, technological advancements, and rising healthcare expenditure. The market, valued at approximately $3426.8 million in 2025, is expected to experience robust growth over the forecast period (2025-2033). This growth is fueled by several key factors. The aging global population is a primary driver, as the incidence of CVDs significantly increases with age. Furthermore, advancements in minimally invasive procedures, improved diagnostic imaging technologies (such as advanced echocardiography and cardiac CT scans), and the development of more sophisticated remote monitoring devices are transforming the landscape, allowing for earlier diagnosis, better treatment, and improved patient outcomes. The increasing adoption of telehealth and remote patient monitoring solutions also contributes to market expansion, enabling continuous monitoring and reducing hospital readmissions. Competition within the market is fierce, with established players like Siemens, GE Healthcare, and Philips Healthcare alongside smaller, innovative companies continually vying for market share.

However, market growth is not without its challenges. High costs associated with advanced diagnostic equipment and procedures can limit accessibility, particularly in developing economies. Furthermore, stringent regulatory approvals and reimbursement policies in various regions can pose barriers to market entry and expansion. Despite these restraints, the long-term outlook remains positive, driven by persistent demand for better CVD diagnostics and management, along with ongoing research and development in the field. The market segmentation is complex, encompassing various device types catering to different diagnostic needs and patient profiles. The ongoing integration of artificial intelligence and machine learning in diagnostic tools promises to further enhance the accuracy and efficiency of CVD detection and management, furthering the growth trajectory.
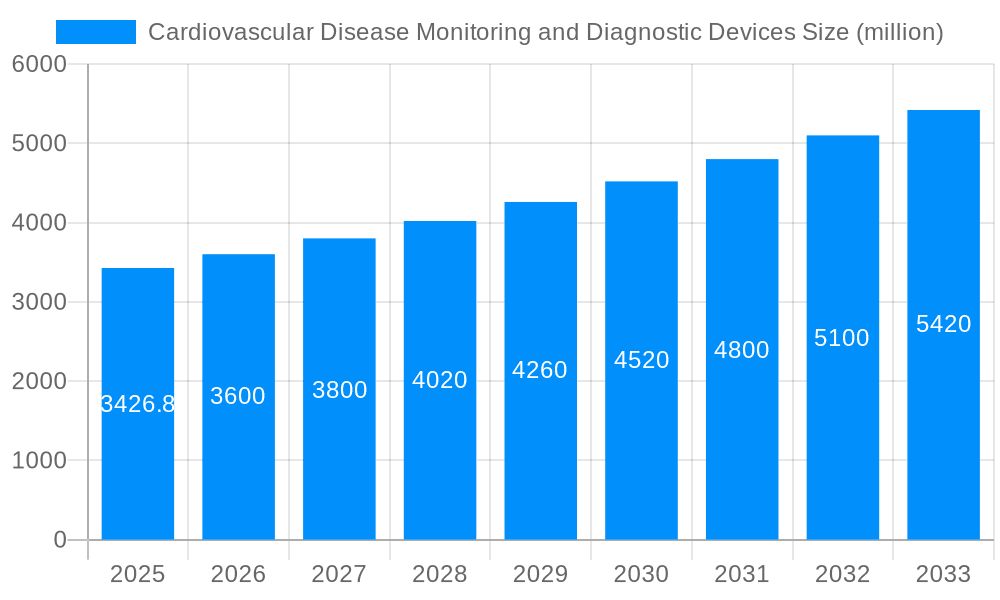

The global cardiovascular disease (CVD) monitoring and diagnostic devices market is experiencing robust growth, driven by the escalating prevalence of cardiovascular diseases worldwide and advancements in medical technology. The market, valued at approximately 25,000 million units in 2025, is projected to exhibit a Compound Annual Growth Rate (CAGR) of X% during the forecast period (2025-2033), reaching an estimated Y million units by 2033. This growth is fueled by several factors, including the increasing geriatric population, rising awareness about CVD risk factors, technological advancements leading to more accurate and minimally invasive diagnostic tools, and the expanding adoption of telehealth solutions for remote patient monitoring. The historical period (2019-2024) already showed significant growth, setting the stage for the even more substantial expansion predicted for the future. Key market insights reveal a strong preference for non-invasive diagnostic techniques and a growing demand for portable and wearable monitoring devices that enable continuous health tracking. The market is also witnessing a shift towards integrated systems that combine multiple diagnostic functionalities into a single platform, thereby streamlining the diagnostic process and improving efficiency. Furthermore, the increasing focus on preventive healthcare and personalized medicine is driving demand for advanced diagnostic tools capable of providing early detection and risk stratification of CVD. This shift necessitates innovative device designs, seamless integration with electronic health records (EHRs), and the development of sophisticated data analytics capabilities to extract actionable insights from the collected patient data. This trend is also impacting the competitive landscape, with manufacturers focusing on developing innovative products and strategic partnerships to capture a larger market share.
Several factors are propelling the growth of the cardiovascular disease monitoring and diagnostic devices market. The aging global population is a significant driver, as the risk of CVD increases with age. This demographic shift leads to a higher demand for diagnostic and monitoring tools. Furthermore, the rising prevalence of lifestyle diseases, such as obesity, diabetes, and hypertension, is significantly increasing the incidence of CVD. These conditions are major risk factors for heart attacks, strokes, and other cardiovascular complications. Technological advancements are another critical factor. Innovations in areas like imaging technology (e.g., improved echocardiography and CT scans), wearable sensors, and implantable devices are enabling earlier and more accurate diagnosis, improved monitoring capabilities, and less invasive treatment options. The increasing adoption of telehealth and remote patient monitoring is also a major growth catalyst. These technologies allow for continuous monitoring of patients' vital signs, early detection of potential complications, and timely intervention, leading to improved patient outcomes and reduced hospital readmissions. Government initiatives aimed at improving cardiovascular health, coupled with rising healthcare expenditure in several countries, are further contributing to the market's expansion. Finally, the increasing awareness about CVD risk factors among the general population is prompting individuals to proactively seek early diagnosis and preventive measures, thereby fueling demand for diagnostic and monitoring devices.
Despite the significant growth potential, the cardiovascular disease monitoring and diagnostic devices market faces several challenges. High costs associated with advanced diagnostic technologies and treatment procedures can pose a significant barrier to access, particularly in low- and middle-income countries. The regulatory landscape governing the approval and use of these devices can be complex and time-consuming, delaying market entry and adoption of new technologies. Moreover, the reimbursement policies for these devices vary across different healthcare systems, potentially affecting their affordability and widespread use. The need for skilled professionals to operate and interpret the data generated by sophisticated diagnostic equipment presents another significant challenge. A shortage of trained personnel can hinder the effective utilization of these devices, especially in regions with limited healthcare infrastructure. Data security and privacy concerns are also emerging as important obstacles. The increasing reliance on digital platforms for data storage and transmission necessitates robust cybersecurity measures to protect sensitive patient information. Finally, the need for continuous innovation and technological advancements to maintain a competitive edge is a constant pressure on manufacturers, requiring significant investment in research and development.
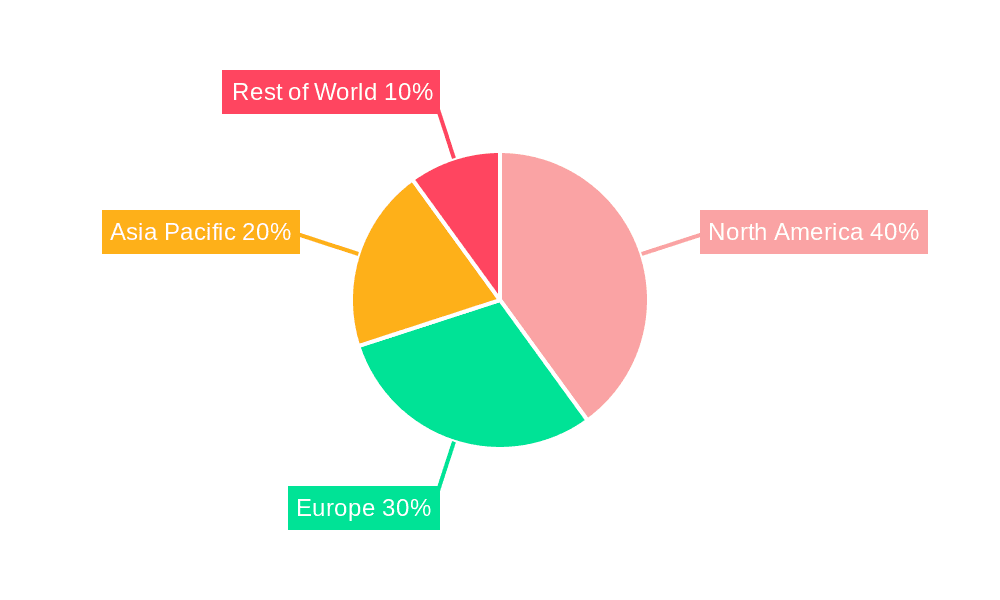
North America: This region is projected to dominate the market due to high healthcare expenditure, technological advancements, and a substantial geriatric population. The presence of major market players and well-established healthcare infrastructure further contributes to its market leadership. The US, in particular, is expected to show substantial growth due to the high prevalence of CVD and the increasing adoption of advanced diagnostic tools.
Europe: Europe holds a substantial market share, driven by government initiatives promoting preventive healthcare and advancements in medical technology across several countries. The growing prevalence of CVD and increasing healthcare expenditure are key growth drivers. Germany, France, and the UK are expected to lead growth within Europe.
Asia Pacific: This region is anticipated to witness significant growth during the forecast period, driven by the rising geriatric population, increasing disposable incomes, and improving healthcare infrastructure. The increasing prevalence of lifestyle diseases contributes to the heightened demand for CVD diagnostic and monitoring devices. Countries like China, India, and Japan are expected to witness rapid expansion.
Segments: The diagnostic imaging segment (including echocardiography, CT scans, MRI) is expected to hold a significant market share due to its high accuracy and detailed insights. The wearable and portable monitoring devices segment is also anticipated to witness rapid growth driven by their convenience and ability to provide continuous monitoring. Finally, the implantable devices segment, while smaller in terms of units sold, will command a significant value due to their high cost and specialized nature.
The market's growth is significantly catalyzed by factors such as technological advancements in diagnostic imaging and wearable sensor technology, allowing for earlier and more accurate diagnosis. Increased investment in research and development is creating sophisticated devices with improved diagnostic capabilities. Government initiatives promoting preventive healthcare and raising awareness about CVD risk factors are creating higher demand for diagnostic and monitoring solutions.
This report provides a detailed analysis of the cardiovascular disease monitoring and diagnostic devices market, encompassing market size estimations, growth projections, and key drivers. It offers insights into the competitive landscape, leading players, and emerging trends. The report also explores various market segments and geographical regions, enabling a comprehensive understanding of market dynamics. The information provided aims to assist businesses and stakeholders in making informed decisions and strategizing for future market opportunities within this crucial healthcare sector.

| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of XX% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include Siemens, Bioanalytical Systems,Inc, Ge Healthcare, Philips Healthcare, Mortara Instrument, Inc, Spacelabs Healthcare Inc., Schiller AG, Hill-Rom, Mindray Medical International Limited, Compumed Inc., Cardionet Inc., Gehealthcare.
The market segments include Type, Application.
The market size is estimated to be USD 3426.8 million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Cardiovascular Disease Monitoring and Diagnostic Devices," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Cardiovascular Disease Monitoring and Diagnostic Devices, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.