1. What is the projected Compound Annual Growth Rate (CAGR) of the Biochips Based In-vitro Diagnostics Market?
The projected CAGR is approximately XXX%.
 Biochips Based In-vitro Diagnostics Market
Biochips Based In-vitro Diagnostics MarketBiochips Based In-vitro Diagnostics Market by End users (Hospitals & Clinics, Research Institutions, Diagnostic Centers), by North America (U.S., Canada, Mexico), by Europe (UK, Germany, France, Italy, Spain, Russia, Netherlands, Switzerland, Poland, Sweden, Belgium), by Asia Pacific (China, India, Japan, South Korea, Australia, Singapore, Malaysia, Indonesia, Thailand, Philippines, New Zealand), by Latin America (Brazil, Mexico, Argentina, Chile, Colombia, Peru), by MEA (UAE, Saudi Arabia, South Africa, Egypt, Turkey, Israel, Nigeria, Kenya) Forecast 2026-2034
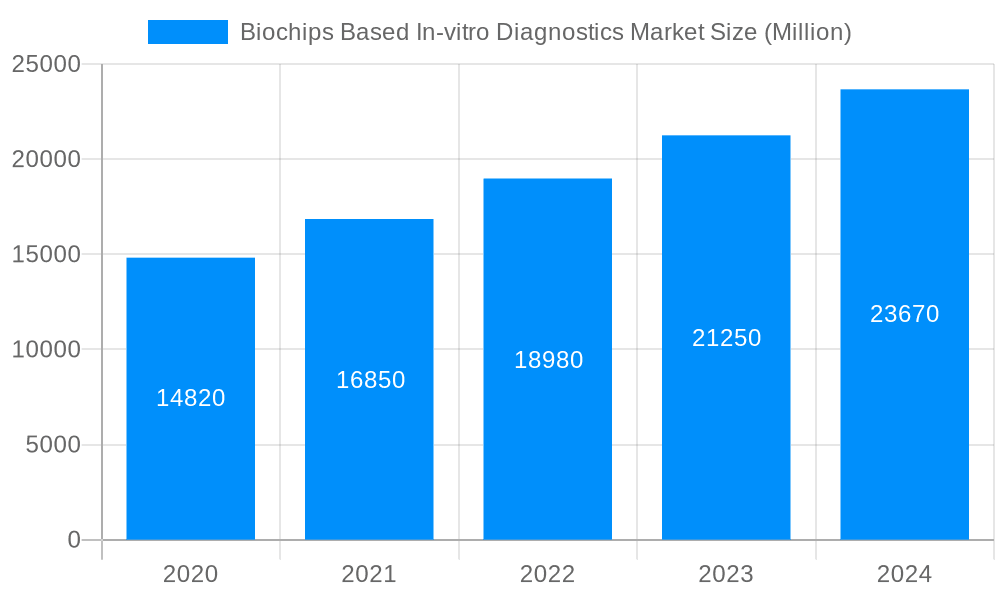
The size of the Biochips Based In-vitro Diagnostics Market was valued at USD XX Million in 2023 and is projected to reach USD XXX Million by 2032, with an expected CAGR of XXX% during the forecast period. Biochips based in-vitro diagnostics (IVD) are advanced diagnostic tools that use miniaturized chips to perform multiple biochemical reactions simultaneously. These chips are integrated with a variety of biological elements such as DNA, proteins, or enzymes, which are used to detect specific biomarkers or pathogens in patient samples. By analyzing small volumes of blood, saliva, or other biological fluids, biochips can rapidly provide diagnostic information on conditions such as infectious diseases, cancer, and genetic disorders. These in-vitro diagnostics offer high precision, speed, and efficiency, enabling personalized medicine and point-of-care testing. Biochips play a critical role in improving disease detection, reducing healthcare costs, and enhancing patient outcomes by allowing for faster and more accurate diagnoses. This robust growth is fueled by a confluence of factors, including the inherent benefits of biochips, such as their miniaturization, high sensitivity, and rapid analysis capabilities. Government initiatives aimed at advancing healthcare infrastructure and promoting precision medicine further bolster the market's growth. Escalating concerns about food security and the pressing need for accurate and timely diagnostics in personalized medicine are also key driving forces. Technological advancements, such as the integration of microfluidics, nanotechnology, and artificial intelligence, are propelling market innovation and expanding the scope of applications.

The demand for biochip-based in-vitro diagnostics is burgeoning due to their inherent advantages over traditional methods. These devices offer superior sensitivity, enabling the detection of biomarkers at extremely low concentrations. Their miniaturization allows for multiplexed assays to be performed simultaneously, reducing time and cost. Additionally, biochips facilitate automation and integration with other analytical platforms, enhancing efficiency and throughput. Furthermore, the growing adoption of point-of-care (POC) diagnostics is driving the demand for portable and user-friendly biochip-based devices that can deliver rapid and accurate results at the patient's bedside or in resource-limited settings.

The adoption of biochips in in-vitro diagnostics is fueled by several key factors:
Despite the promising potential of biochips in in-vitro diagnostics, the market also faces certain challenges and restraints:
The Biochips Based In-vitro Diagnostics Market is witnessing significant growth across different regions and segments:
The Biochips Based In-vitro Diagnostics Industry is experiencing a period of dynamic expansion, fueled by a confluence of transformative technological advancements and increasing global demand for more precise and accessible diagnostic solutions. The key drivers propelling this growth include:
The Biochips Based In-vitro Diagnostics Market is characterized by a competitive landscape with several leading players:
The Biochips Based In-vitro Diagnostics Sector is characterized by rapid innovation and strategic collaborations, with several noteworthy advancements shaping its trajectory:
The comprehensive coverage Biochips Based In-vitro Diagnostics Market Report provides detailed insights into the market's:
The detailed Report of Biochips Based In-vitro Diagnostics Market encompasses the following aspects:
The Report provides a comprehensive analysis of the pricing trends in the Biochips Based In-vitro Diagnostics Market, including:
The Report offers a detailed analysis of the import and export trends in the Biochips Based In-vitro Diagnostics Market, covering:
The Report provides a comprehensive analysis of the patent and trademark landscape in the Biochips Based In-vitro Diagnostics Market, including:
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of XXX% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XXX%.
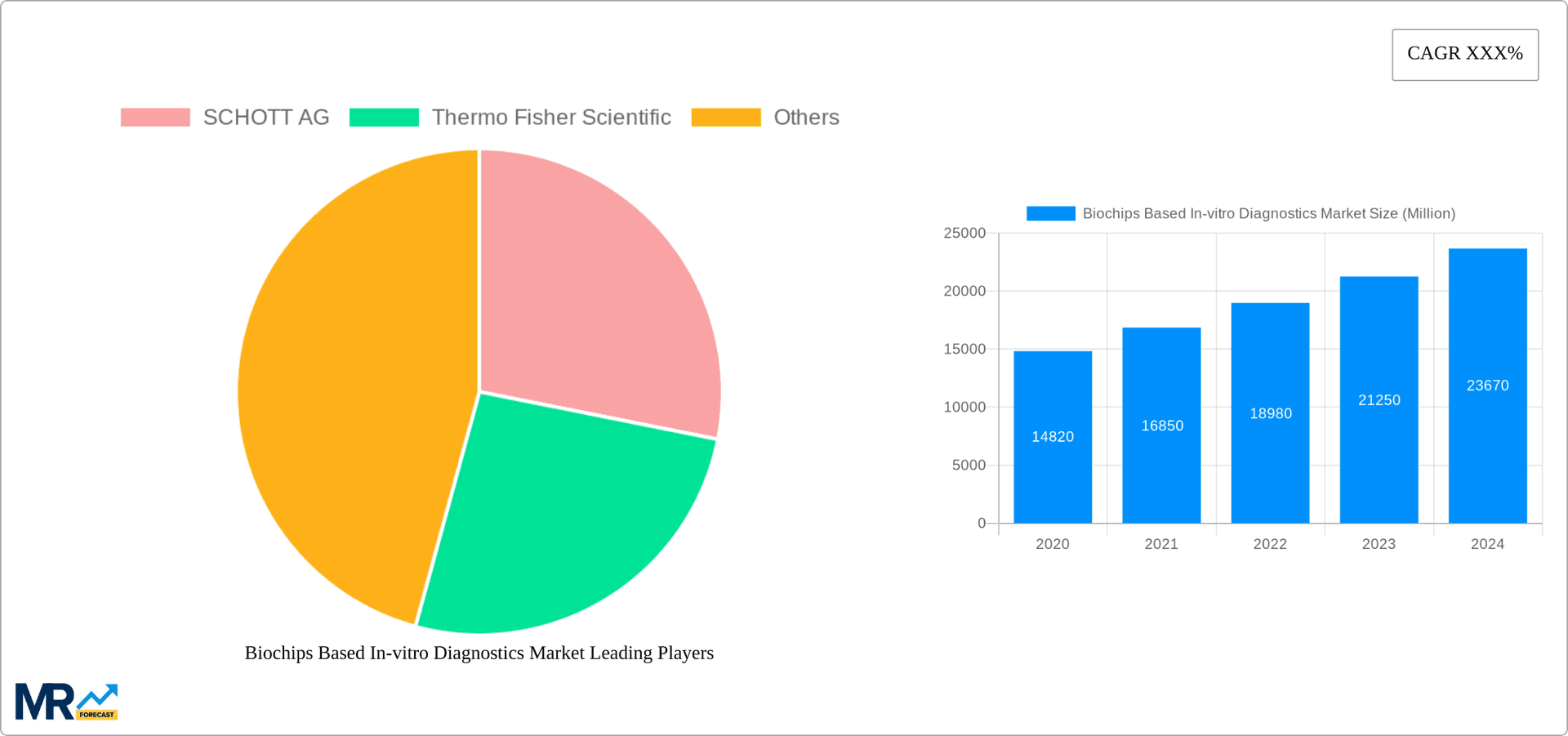
Key companies in the market include SCHOTT AG, Thermo Fisher Scientific.
The market segments include End users.
The market size is estimated to be USD XX Million as of 2022.
N/A
N/A
N/A
In March 2019, Axbio Inc. announced the successful development of the company’s In-Vitro Diagnostic Bio-CMOS IC based on the collaborator TowerJazz’s Advanced 300mm 65nm RFCMOS Platform. The peer reviewed journal, The Lancet, published by Elsevier announced in March, 2019 that ongoing R&D is being conducted for the rapid and accurate diagnosis of the respiratory disease, pertussis, on a point-on-care biochip.
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4850, USD 5850, and USD 6850 respectively.
The market size is provided in terms of value, measured in Million.
Yes, the market keyword associated with the report is "Biochips Based In-vitro Diagnostics Market," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Biochips Based In-vitro Diagnostics Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.