1. What is the projected Compound Annual Growth Rate (CAGR) of the Adhesives for Wearable Medical Device?
The projected CAGR is approximately 13.5%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Adhesives for Wearable Medical Device
Adhesives for Wearable Medical DeviceAdhesives for Wearable Medical Device by Application (Diagnostic Device, Monitoring Device, Drug Delivery Devices), by Type (Acrylics Based, Silicone Based, Others), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
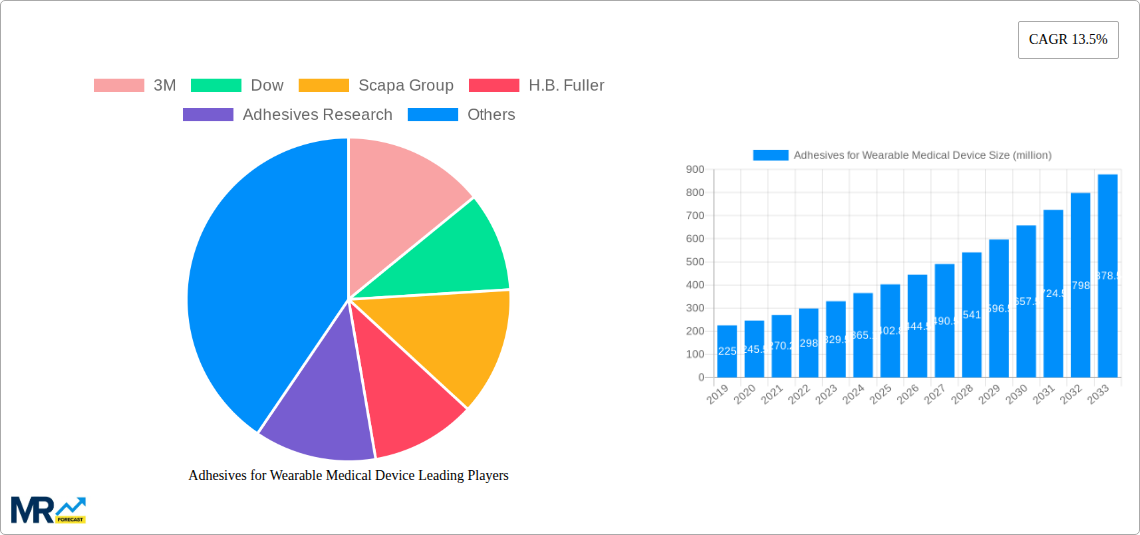
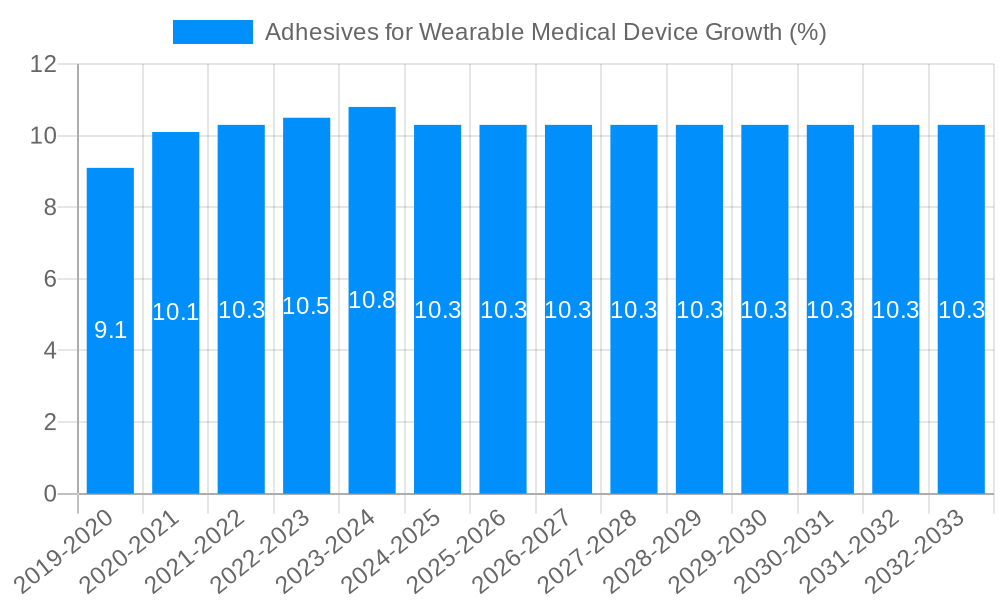
The global market for Adhesives for Wearable Medical Devices is poised for robust expansion, projected to reach USD 402.8 million by 2025 and exhibiting a Compound Annual Growth Rate (CAGR) of 13.5% through 2033. This significant growth is primarily fueled by the escalating demand for advanced diagnostic and monitoring devices, driven by an aging global population and a rising prevalence of chronic diseases. The increasing adoption of telehealth and remote patient monitoring solutions further amplifies the need for reliable, skin-friendly adhesives that ensure device adherence and user comfort during extended wear. Furthermore, the continuous innovation in adhesive technologies, particularly in developing biocompatible and flexible materials, is a key catalyst, enabling the creation of smaller, more discreet, and highly functional wearable devices for applications ranging from continuous glucose monitoring and ECG tracking to sophisticated drug delivery systems.
The market is segmented by application, with Diagnostic Devices and Monitoring Devices expected to lead the charge due to their widespread use in chronic disease management and preventative healthcare. Drug Delivery Devices, while a smaller segment, present a high-growth opportunity as the trend towards transdermal delivery of therapeutics gains momentum. In terms of type, Acrylics-Based and Silicone-Based adhesives are anticipated to dominate owing to their excellent biocompatibility, flexibility, and adhesion properties, crucial for direct skin contact. Key players such as 3M, Dow, Scapa Group, and Henkel are at the forefront of this market, investing heavily in research and development to introduce next-generation adhesive solutions that meet the stringent regulatory requirements and evolving needs of the wearable medical device industry. The Asia Pacific region, particularly China and India, is expected to witness the fastest growth due to increasing healthcare expenditure, a burgeoning medical device manufacturing sector, and a growing awareness of wearable health technologies.
This comprehensive report delves into the dynamic and rapidly expanding global market for adhesives used in wearable medical devices. Spanning a meticulous study period from 2019 to 2033, with a base year set in 2025, this analysis provides an in-depth understanding of market dynamics, key drivers, prevailing challenges, and future growth trajectories. Leveraging extensive data from the historical period (2019-2024) and robust projections for the forecast period (2025-2033), this report equips stakeholders with invaluable insights to navigate this critical sector. The estimated market size for 2025 is projected to be [Insert Specific Value in Millions, e.g., 1,250 million USD], with significant growth anticipated.
The adhesives market for wearable medical devices is experiencing a transformative surge, driven by the relentless pursuit of enhanced patient comfort, improved diagnostic accuracy, and seamless integration of technology into daily life. The increasing prevalence of chronic diseases and the aging global population are powerful catalysts, fueling the demand for continuous monitoring and personalized treatment solutions that wearable devices epitomize. Furthermore, the shift towards remote patient monitoring and telehealth services, significantly accelerated by recent global events, has placed wearable medical devices at the forefront of healthcare delivery. This trend necessitates adhesives that offer superior biocompatibility, extended wear times, and robust adhesion to diverse skin types and varying physiological conditions, including perspiration and body oils. Innovation in adhesive formulations is increasingly focused on biocompatible and hypoallergenic materials, moving away from traditional irritants and towards advanced polymers like medical-grade silicones and specialized acrylics. The demand for thinner, more flexible, and less obtrusive adhesive solutions is also on the rise, enabling the development of discreet and comfortable wearable devices that patients are more likely to adhere to long-term. Smart wearables with integrated sensors for glucose, vital signs, and even electrophysiological monitoring are becoming mainstream, requiring adhesives capable of maintaining consistent contact and signal integrity over extended periods. The concept of "skin-like" adhesives, offering breathability and conforming to body contours with minimal disruption to the skin barrier, is gaining traction. This evolution is crucial for reducing skin irritation, enhancing user experience, and ultimately improving patient compliance and therapeutic outcomes. The integration of advanced functionalities within wearable devices, such as miniaturized drug delivery systems or sophisticated diagnostic sensors, is also pushing the boundaries of adhesive technology, demanding materials that can withstand various environmental factors and biological interactions.
Several potent forces are collectively driving the expansion of the adhesives market for wearable medical devices. Foremost among these is the burgeoning demand for remote patient monitoring. As healthcare systems worldwide grapple with increasing costs and the need for proactive disease management, wearable devices offer a compelling solution for continuous data collection, allowing for early detection of health anomalies and personalized interventions. This surge in remote monitoring directly translates into a heightened need for reliable, skin-compatible adhesives that can secure these devices for extended durations. Coupled with this is the escalating global burden of chronic diseases such as diabetes, cardiovascular conditions, and respiratory ailments. Wearable technologies provide an invaluable tool for managing these conditions through consistent tracking of vital signs, glucose levels, and activity patterns, thereby necessitating a robust supply of specialized medical adhesives. The accelerating pace of technological innovation in the medical device sector is another significant propellant. Miniaturization of sensors, development of advanced bio-integrated electronics, and the integration of therapeutic functions within wearable devices are all creating new adhesive application requirements. These advancements demand adhesives that offer not only strong and secure attachment but also electrical conductivity, thermal management, and compatibility with complex electronic components. Furthermore, a growing consumer awareness and acceptance of wearable technology for health and wellness purposes, coupled with increasing disposable incomes in developed and emerging economies, contribute to a broader market adoption, further stimulating demand for the underlying adhesive components.
Despite the robust growth, the adhesives market for wearable medical devices is not without its formidable challenges and restraints. One of the most critical concerns is ensuring stringent biocompatibility and minimizing the risk of skin irritation or allergic reactions. The human skin is a sensitive organ, and adhesives must undergo rigorous testing and regulatory approval processes to meet global standards, which can be time-consuming and costly. This necessitates the use of specialized, often more expensive, raw materials. Another significant challenge lies in achieving the delicate balance between strong adhesion and the ability to remove the device without causing skin trauma or residue. Wearable devices are often worn for extended periods, and the adhesive must maintain its integrity through sweat, movement, and varying environmental conditions, yet peel off cleanly when needed. This requires sophisticated adhesive formulations that adapt to these dynamic conditions. The cost of developing and manufacturing high-performance, medical-grade adhesives can also be a restraint, particularly for smaller device manufacturers or in price-sensitive markets. The complex supply chain for medical-grade raw materials and the stringent quality control measures add to the overall production costs. Furthermore, the rapid pace of technological evolution in wearable devices means that adhesive manufacturers must constantly innovate to keep pace with new material requirements, such as the need for adhesives that can conduct electricity, dissipate heat, or be integrated with advanced sensor technologies, posing a continuous research and development challenge.
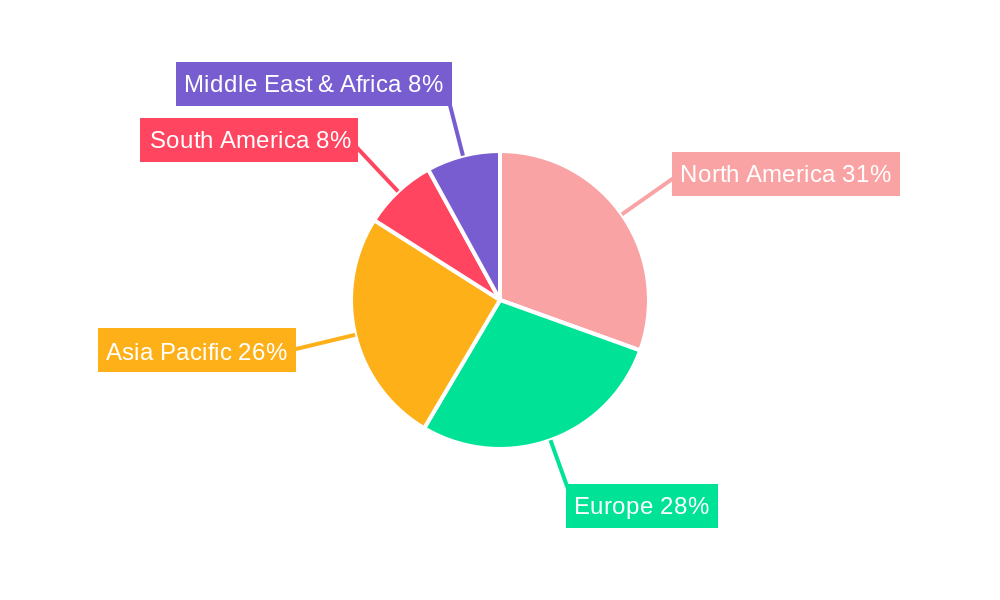
The global adhesives for wearable medical device market is characterized by dominant regions and segments that are shaping its trajectory. Geographically, North America, particularly the United States, and Europe stand out as key regions poised to dominate the market. This dominance is attributed to several converging factors: a well-established healthcare infrastructure, high adoption rates of advanced medical technologies, a significant aging population, and a strong emphasis on proactive health management and chronic disease care. The presence of leading medical device manufacturers, robust research and development capabilities, and favorable regulatory environments further solidify their leadership. The United States, with its substantial healthcare expenditure and a thriving innovation ecosystem, is a prime example of a market where the demand for sophisticated wearable medical devices, and consequently the adhesives that enable them, is exceptionally high. Similarly, European countries, with their universal healthcare systems and a growing focus on elder care and personalized medicine, present substantial opportunities.
Within these leading regions, the Monitoring Device segment is projected to be a major growth driver and likely a dominant application. The increasing prevalence of chronic conditions such as diabetes, hypertension, and cardiac arrhythmias necessitates continuous, non-invasive monitoring of physiological parameters. Wearable devices, including continuous glucose monitors (CGMs), smartwatches with ECG capabilities, and wearable blood pressure monitors, are becoming indispensable tools for both patients and healthcare providers. The adhesives used in these devices must ensure reliable skin contact for extended periods (days to weeks) to facilitate accurate and uninterrupted data collection. This requires adhesives with exceptional biocompatibility, excellent adhesion to diverse skin types even under challenging conditions like perspiration, and minimal skin irritation. The development of advanced, transparent, and breathable adhesive films is critical for enhancing patient comfort and compliance in this segment.
In terms of adhesive Type, Acrylics Based adhesives are expected to hold a significant market share and play a crucial role in market dominance. Acrylics offer a versatile platform for developing a wide range of medical adhesives, providing a good balance of adhesion strength, flexibility, and cost-effectiveness. Their ability to be formulated into pressure-sensitive adhesives (PSAs) makes them ideal for applications requiring easy application and removal, as well as long-term wear. For wearable medical devices, acrylic-based adhesives can be engineered to provide excellent tack, shear strength, and cohesion, ensuring the device remains securely in place. They are also amenable to various sterilization methods. While silicone-based adhesives are gaining traction due to their superior biocompatibility and flexibility, particularly for sensitive skin applications and high-moisture environments, acrylics continue to offer a more cost-effective solution for many standard monitoring and diagnostic devices. The ongoing innovation in acrylic polymer chemistry is further enhancing their performance characteristics, making them increasingly competitive and adaptable to the evolving demands of wearable medical device technology, thus positioning them as a dominant type in the foreseeable future.
Several key growth catalysts are propelling the adhesives for wearable medical device industry forward. The increasing global prevalence of chronic diseases such as diabetes and cardiovascular conditions necessitates continuous, long-term monitoring, directly driving demand for reliable and skin-friendly adhesives. Furthermore, the accelerating adoption of telehealth and remote patient monitoring solutions, amplified by recent global health events, positions wearable devices as essential components of modern healthcare, thus boosting adhesive consumption. The rapid advancements in miniaturization and sensor technology within the medical device sector are also creating new opportunities for specialized adhesive formulations capable of integrating with complex electronic components and ensuring optimal signal transmission.
This report offers an exhaustive examination of the global adhesives for wearable medical device market, providing a holistic view of its current landscape and future potential. It meticulously analyzes market size and forecast from 2019 to 2033, with a detailed focus on the estimated value for 2025. The analysis covers key segments including Application (Diagnostic, Monitoring, Drug Delivery Devices), Type (Acrylics Based, Silicone Based, Others), and critically examines Industry Developments. The report thoroughly investigates the driving forces, challenges, and restraints shaping the market, alongside identifying dominant regions and countries, and key segments within the market, offering deep insights into their market share and growth drivers. Furthermore, it highlights crucial growth catalysts and provides a comprehensive list of leading market players. This report is an indispensable resource for stakeholders seeking to understand market dynamics, identify growth opportunities, and formulate effective business strategies in the rapidly evolving field of wearable medical device adhesives.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 13.5% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 13.5%.
Key companies in the market include 3M, Dow, Scapa Group, H.B. Fuller, Adhesives Research, Henkel, Vancive Medical Technologies, Lohmann, Elkem Silicones, Polymer Science, Inc., Adhezion Biomedical, .
The market segments include Application, Type.
The market size is estimated to be USD 402.8 million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million.
Yes, the market keyword associated with the report is "Adhesives for Wearable Medical Device," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Adhesives for Wearable Medical Device, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.